GSK3b explained
In this post I will attempt to explain the role of the GSK3b protein in layman terms. For this I will get some help from ChatGPT :)
What is GSK3b
Glycogen Synthase Kinase 3β (GSK3β) is an enzyme found in the body that plays an important role in many different processes. GSK3β has two forms, α and β, with β being more important in the human brain. The activity of GSK3β can be turned on or off by different chemicals in the body. When GSK3β is turned on, it affects many things in the body such as energy metabolism, cell survival, and neurotransmission. GSK3β is regulated by a lot of different enzymes and proteins that work together to control its activity. Some of these chemicals turn off GSK3β, while others turn it on.
When GSK3β is turned off, it can be a result of chemicals like insulin or growth factors, including WNT signaling. These chemicals work by attaching to certain proteins that then turn off GSK3β. Other chemicals that can turn off GSK3β include cytokines and other growth factors.
When GSK3β is turned on, it can be a result of chemicals like calcium, protein kinase A, or other signaling pathways. These chemicals work by attaching to different proteins that then turn on GSK3β.
Overall, GSK3β is an important enzyme in the body that helps to control many different processes. By understanding how GSK3β works, scientists can better understand how the body functions and how to treat different diseases.
Neurotransmission
GSK3β is affected by different chemicals in the body, including neurotransmitters, which are chemicals that transmit signals in the brain. Here are some examples of how GSK3β is affected by different types of neurotransmitters:
-
Glutamate: This is the most abundant excitatory neurotransmitter in the brain. It acts through AMPA and NMDA receptors, which are involved in membrane depolarization and neuroplasticity, respectively. The activity of NMDA receptors leads to calcium-dependent protein kinase 2 (CaMK2) activation, which inhibits GSK3β. In contrast, modest calcium concentration activates calcineurin, which activates GSK3β.
-
GABA: This is the main inhibitory neurotransmitter in the brain. GABAergic transmission inhibits GSK3β by acting through GABAB receptors. On the other hand, GSK3β phosphorylates gephyrin, which leads to the formation of new GABA receptors. (R2)
-
Dopamine: This neurotransmitter is involved in motor control, motivation, reward, and executive functions. The effects of its signaling on GSK3β depend on the type of dopamine receptors expressed on the neuronal surface. Stimulation of D1R and D3R inhibits GSK3β (via Akt), while activation of D2R leads to GSK3β activation (via PP2A).
-
Serotonin: Stimulation of 5-HT1 and 5-HT7 receptors activates the PI3K/AKT pathway and increases Ser9 phosphorylation in GSK3β, while activation of 5-HT2A receptor has the opposite effect.
-
Noradrenaline: This neurotransmitter inhibits GSK3β activity by acting through α1A, α2, and β-adrenergic receptors.
In summary, GSK3β activity is related to the downsizing of synapses and decreased excitability of neurons. Inhibition of GSK3β is necessary for the induction of long-term potentiation (LTP), the process underlying new memory formation. The activity of GSK3β is affected by different neurotransmitters in various ways, which can affect many processes in the brain.
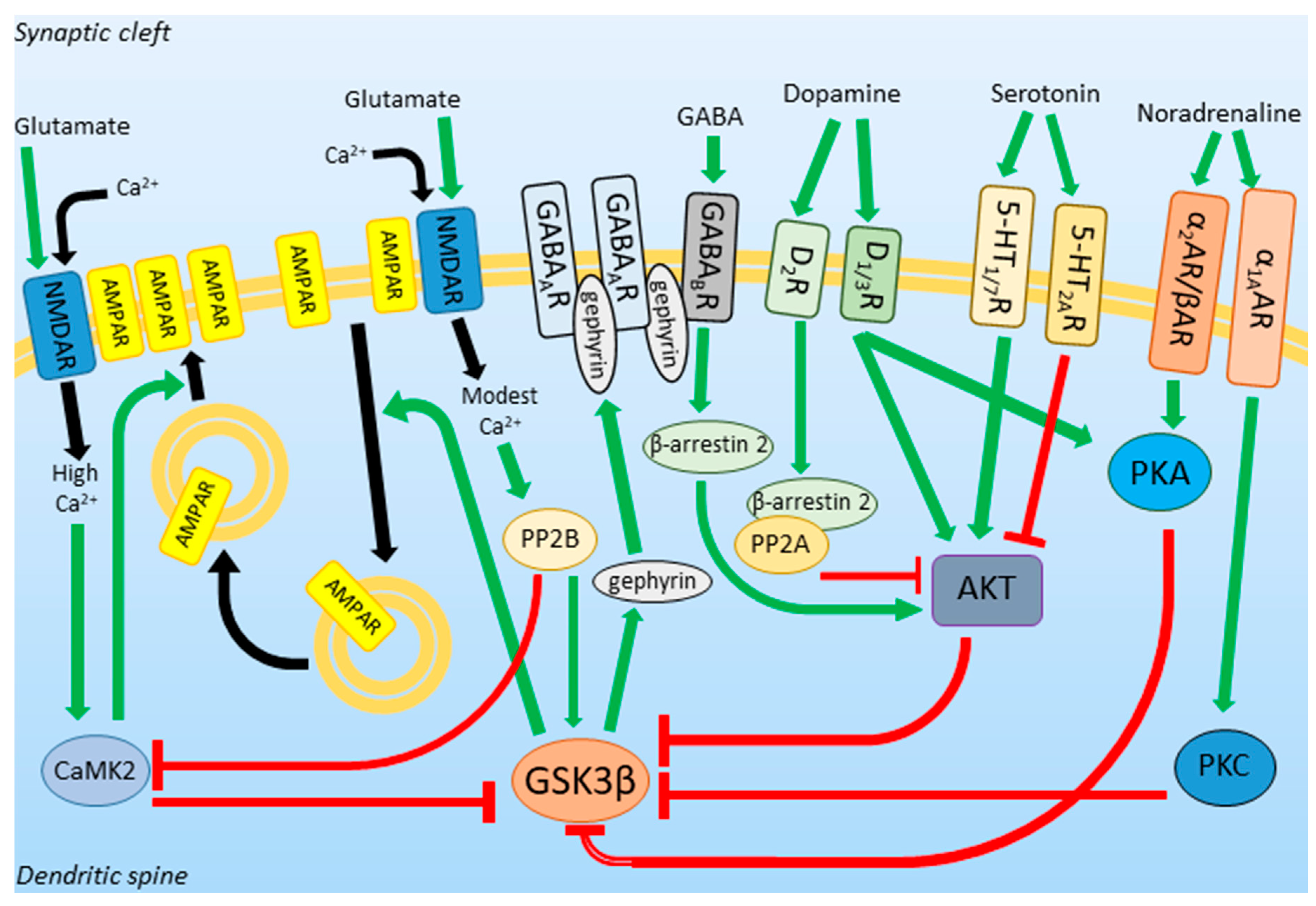
Text is based on the Section 1.3 of the paper, rewritten by chatGPT and validated by me afterwards. Figure is borrowed from the paper directly.
Role in depression and anxiety
The role of GSK3β in the pathogenesis of major depressive disorder (MDD) is complex and involves many cellular processes and factors. While the current pharmacotherapy of MDD is based on the monoaminergic hypothesis, various animal models of depression have been established that suggest a more multifaceted etiology. GSK3β, an enzyme that is involved in numerous signaling pathways related to neurotrophic and pro-inflammatory factors, is likely one of the key factors involved in MDD onset.
Studies on animal models of depression have revealed the importance of GSK3β activity in the development of manic-like disturbances and stress-induced depressive-like behavior. For example, GSK3α/β knock-in mice (i.e. GSK3 is permanently activated) have exhibited heightened responses to novel environments and increased susceptibility to stress-induced depressive-like behavior. They also displayed mild-anxious behavior, which often coexists with depression. Additionally, studies have shown that increased activity of GSK3β is sufficient to impair mood regulation in learned helplessness models of depressive-like behavior.
Interestingly, there is also evidence to suggest that GSK3β activity is involved in anxiety. For instance, the GSK3β knock-in mice mentioned above showed anxiety-like behavior in the elevated plus maze, indicating that GSK3β activity is related to anxiety as well as depression.
Furthermore, studies have shown that GSK3β inhibition can have an antidepressant-like effect. For example, bilateral intra-hippocampal injections of lentivirus expressing shRNA anti-Gsk3β induced an antidepressant-like effect in chronically stressed mice.
Heterozygous loss of Gsk3β also caused behavioral defects that mimic the action of lithium, a classical mood stabilizer, and transgenic expression of Gsk3β in Gsk3β+/− heterozygotes reversed these defects. The same effect was observed when GSK3β was overexpressed in lithium-treated mice. These findings strongly support the hypothesis that the MDD phenotype is due to specific inhibition of the kinase.
In summary, GSK3β is involved in numerous signaling pathways related to neurotrophic and pro-inflammatory factors, and its altered activity is likely involved in the complex etiology of MDD. Animal studies have shown that GSK3β activity is related to both anxiety and depression, and that its inhibition can have an antidepressant-like effect.
BDNF
BDNF (brain-derived neurotrophic factor) is a protein that supports the growth, development, and survival of neurons in the brain.
Altered BDNF activity may be linked to changes in brain structure and behavior associated with depression and anxiety.
BDNF supports neuron survival and growth, and its effects on GSK3β activity are well characterized. In depression, BDNF levels are reduced in the PFC and hippocampus, but increased in mesolimbic structures. The BDNF-dependent inhibition of GSK3β activity is required for dendritic growth and arborization, and reduced GSK3β inhibition may be related to depression-like behavior.
The downstream target of BDNF, p11, is positively regulated by BDNF and has anti-depressant-like effects(R3). BDNF induces protein synthesis via mTOR, which is inhibited by REDD1. Chronic stress increases REDD1 and reduces mTOR levels and protein synthesis(R4), and GSK3β may trigger REDD1 ubiquitination and degradation by the proteasome.
Unfolded protein response
Recent research has established a link between depression and the unfolded protein response (UPR), which is a cellular stress response mechanism triggered by the buildup of misfolded proteins in the endoplasmic reticulum (ER). The UPR is activated in an attempt to restore ER homeostasis, but if that’s not possible, apoptosis may occur.
Studies have found signs of ER-stress-induced UPR in the brains of depressed patients who died by suicide, indicating a strong connection between depression and UPR. UPR activation has also been observed in tauopathies, where GSK3β is known to be involved in tau phosphorylation. As a result, the role of GSK3β in ER-stress-induced UPR has been extensively studied.
It has been discovered that during UPR activation, the activity of GSK3β, which plays a role in regulating CHOP protein expression, increases due to reduction of phosphorylation of Ser9.
It has been proposed that GSK3β is strongly connected with the UPR, which could be one of the potential causes of depression. In addition, UPR has also been shown to be active in the mitochondria of a murine model of depression, pointing to a new mechanism involved in the development of the disease.
Role in circadian rhythm
GSK3 affects at least five core clock proteins and GSK3b inactivation has diurnal variation. Chronic activation of GSK3 impaired rhythmicity of BMAL1 (which is a target for GSK3).
Furthermore, chronic pharmacological inhibition of GSK3 with 20 μM CHIR-99021 enhanced the amplitude and shortened the period of PER2::luciferase rhythms in organotypic SCN slice cultures. These results support the model that GSK3 activity status is regulated by the circadian clock and that GSK3 feeds back to regulate the molecular clock amplitude in the SCN. (R5)
Previous evidence suggests that GSK3 directly phosphorylates at least five core clock proteins: PER2, CRY2, CLOCK, BMAL1, and REVERBα)
Activating proteins
- Protein kinase A (PKA)
- Integrin-linked kinase (ILK)
- Calcium/calmodulin dependent protein kinase 2 (CaMK2)
- Protein phosphatase 1 (PP1)
- Protein phosphatase 2A (PP2A)
- Protein phosphatase 2B (PP2B, calcineurin)
Inhibiting signals
- Insulin
- Insulin-like growth factor 1 (IGF-1)
- Epidermal growth factor (EGF)
- Platelet-derived growth factor (PDGF)
- Transforming growth factor 1β (TGF-1β)
- Mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathway
- Fibroblast growth factor (FGF)
- Nerve growth factor (NGF)
- Brain-derived neurotrophic factor (BDNF)
- Cytokines
- WNT