Melatonin
Melatonin as a pro-oxidant
Melatonin is usually regarded as an antioxidant and a protective agent. Here I provide a few pieces of research that show that Melatonin can be also a pro-oxidant depending on the level of oxidative stress.
In yeast, melatonin was shown to reduce GSH and increase GSSG at the same time activating some genes related to antioxidant defense system and downregulating other genes:
Melatonin alone decreased GSH, increased GSSG, and activated antioxidant defense system genes, which reached maximum levels in the stationary phase.
These results indicate that melatonin supplementation enables cells to resist better the stress generated in the stationary phase.
However, when cells were subjected to oxidative stress induced by H2O2, melatonin was able to partially mitigate cell damage by decreasing ROS accumulation and GSH and increasing GSSG [R1]
It seems the protective mode of action here is to slow down metabolism by reducing GSH/GSSG ratio.
Significant downregulation of Thioredoxin
What is surprising and possibly very important is that melatonin significantly reduced expression of cytosolic Thioredoxin (see Figure 3 in the original paper):
Upon entry into the stationary phase (16 h), expression of all genes increased significantly under the control condition (Figure 3), with the highest levels found for catalase genes (CTT1 and CTA1; 1000 and 600 times, respectively) and the lowest for GLR1 and ZWF1 (2 and 3 times, respectively).
Moreover, at this time point, the presence of MEL resulted in a greater increase in the expression level of most genes (GSH1, GPX1, GLR1, CTA1, SOD1, SOD2, and GRX2), between 3 and 5 times higher than under the control condition (Figure 3, inset).
Exceptions were CTT1 and ZWF1, expression of which was similar to the control, and TRX2, expression of which remained lower than the control. [R1]
TRX2 here is a cytosolic thioredoxin. In humans cytosolic thioredoxin Txn1 is encoded by the TXN gene.
Figure 3 shows that:
- 6 hours after applying MEL expression of GSH1, GPX1 and TRX2 is reduced. TRX2 is reduced significantly.
- 16 hours after applying MEL levels of most enzymes is increased significantly, except for TRX2 - its level is still lower than in control.
It seems MEL has long lasting effect on TRX2 in yeast.
Thioredoxin is reduced In humans too?
Another paper that studied the effect on proteins in human placenta cells shows interesting results about Thioredoxin - while mRNA level of TXN increased, the level of protein seems to be reduced (in the raw data of the study), but the authors concluded “no difference”:
Melatonin increased TXN mRNA expression at the 10μM concentration (Fig 1M; see S2 File).
Consistently NQO1 protein was not increased in HUVECs with melatonin treatment, however while TXN mRNA was increased in HUVECs treated with melatonin (Fig 1M; see S2 File) there was no change in TXN protein in HUVECs treated with melatonin (Fig 1O; see S2 File).
Here is a snapshot of the measurements of TXN density from the supplemental file of the study:
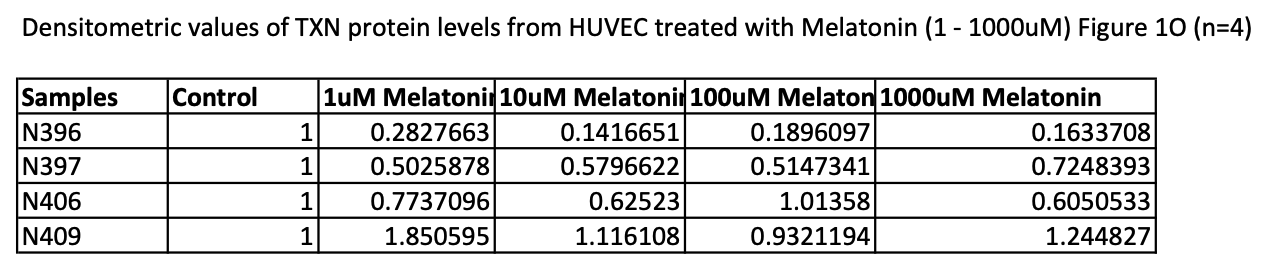
In the first three samples the level of TXN protein is lower than in the control group. Only the sample N409 is very different with higher TXN levels.
Melatonin in U937 cells
Third paper shows significant prooxidant properties of Melatonin:
In contrast to other antioxidant molecules which decrease intracellular ROS concentration, melatonin significantly increases intracellular ROS formation following 2 h and 6 h of incubation with U937 cells (FIG. 1).
The data also indicate that at 2 h of incubation intracellular ROS are strongly increased (more than sevenfold) while after 6 h the variation is less consistent (about threefold).
The increase of oxidative species is accompanied by a depletion of U937 intracellular GSH concentration during melatonin treatment. In fact, at 2 h and at 6 h of incubation, intracellular GSH concentration is 73.3% and 65.5% of the control, respectively (FIG. 2). R3
4th study also demonstrates ROS-generating property of melatonin with a remark that it does NOT lead to oxidative stress:
Here we show that on U937 human monocytes melatonin promotes intracellular ROS in a fast (<1 min) and transient (up to 5-6 h) way.
Melatonin equally elicits its pro-radical effect on a set of normal or tumor leukocytes;
intriguingly, ROS production does not lead to oxidative stress, as shown by absence of protein carbonylation, maintenance of free thiols, preservation of viability and regular proliferation rate. R4
Melatonin-calmodulin interaction
Another study demonstrates that:
- Binding of melatonin to calmodulin is likely the key to ROS production
- Calmodulin binds to phospholipase A2 which deactivates the enzyme. Without calmodulin PLA2 produces ROS and Arachidonic acid.
- Melatonin protects PLA2 from destruction, which leads to increased ROS and AA formation.
We have shown that melatonin immediately and transiently stimulates intracellular free radical production on a set of leukocytes, possibly as a consequence of calmodulin binding.
We show here that melatonin-induced ROS are produced by lipoxygenase (LOX), since they are prevented by a set of LOX inhibitors, and are accompanied by increase of the 5-LOX product 5-HETE.
LOX activation is accompanied by strong liberation of AA;
inhibition of Ca(2+)-independent, but not Ca(2+)-dependent, phospholipase A2 (PLA2), prevents both melatonin-induced arachidonic acid and ROS production, whereas LOX inhibition only prevents ROS, indicating that PLA2 is upstream with respect to LOX, as occurs in many signaling pathways.
Chlorpromazine, an inhibitor of melatonin-calmodulin interaction, inhibits both ROS and arachidonic acid production, thus possibly placing calmodulin at the origin of a melatonin-induced pro-radical pathway.
Interestingly, it is known that Ca(2+)-independent PLA2 binds to calmodulin: our results are compatible with PLA2 being liberated by melatonin from a steady-state calmodulin sequestration, thus initiating an arachidonate signal transduction. R5
Induction of RET
Here we report that melatonin mediates apoptosis in head and neck cancer by driving mitochondrial reverse electron transport (RET) to induce ROS production.
Melatonin‐induced changes in tumoral metabolism led to increased mitochondrial activity, which, in turn, induced ROS‐dependent mitochondrial uncoupling. Interestingly, mitochondrial complex inhibitors, including rotenone, abolished the ROS elevation indicating that melatonin increased ROS generation via RET.
Melatonin also increased membrane potential and CoQ10H2/CoQ10 ratio to elevate mitochondrial ROS production, which are essential conditions for RET.
We found that genetic manipulation of cancer cells with alternative oxidase, which transfers electrons from QH2 to oxygen, inhibited melatonin‐induced ROS generation, and apoptosis.
RET restored the melatonin‐induced oncostatic effect, highlighting the importance of RET as the site of ROS production. (R6)
Induction of synthesis
On the other hand, darkness activates these noradrenergic neurons with a consequent release of noradrenaline and activation of specific receptors present on the pineal cells that, in turn, stimulate the production of N-acetyltransferase and the biosynthesis of melatonin (R7)
Metabolism of melatonin
Melatonin is metabolized in the human organism in the liver, skin, lung and brain through the CYP1A1, CYP1A2 and CYP1B1 isozymes.
Endogenous and exogenous melatonin is degraded to 6-hydroxymelatonin and N-acetylserotonin by aromatic hydroxylation and O-demethylation, respectively.
…
The various P450 isozymes react differently with melatonin, namely CYP1A1, CYP1A2 and CYP1B1 give dominant 6-hydroxylation, while CYP2C19 produces O-demethylation products selectively. Nevertheless, with CYP1A2 some O-demethylation products are also observed. (R8)
Melatonin inhibits GSK3b
Melatonin has found to reduce the GSK3β mRNA expression and protein level by western blot and immunofluorescence assay. Melatonin has also decreased phospho-Tau level (pThr181 and pThr212-pSer214) in neuron cell line upon OA induction as seen by microscopic analysis. (R9)